UNMATCHED QUALITY AND PURITY
Quality Beyond Question: A Deep Dive into Our Stem Cell Testing Results
At GainMed, excellence in stem cell products isn’t just a goal—it’s our baseline. We achieve this through a relentless commitment to quality, underscored by extensive testing and absolute transparency. Below, we present a snapshot of our batch testing results, accompanied by a detailed explanation that underscores the exceptional quality and purity that define our products.
Stimulating Growth: They contain key signals that can awaken dormant hair follicles, encouraging the transition from the telogen (resting) phase to the anagen (growth) phase.
Anti-inflammatory Properties: Exosomes have inherent anti-inflammatory properties that can reduce scalp inflammation, a common contributing factor to hair loss.
Promoting Blood Supply: By enhancing angiogenesis, exosomes help improve blood flow to the scalp, providing follicles with the necessary nutrients and oxygen for hair growth.
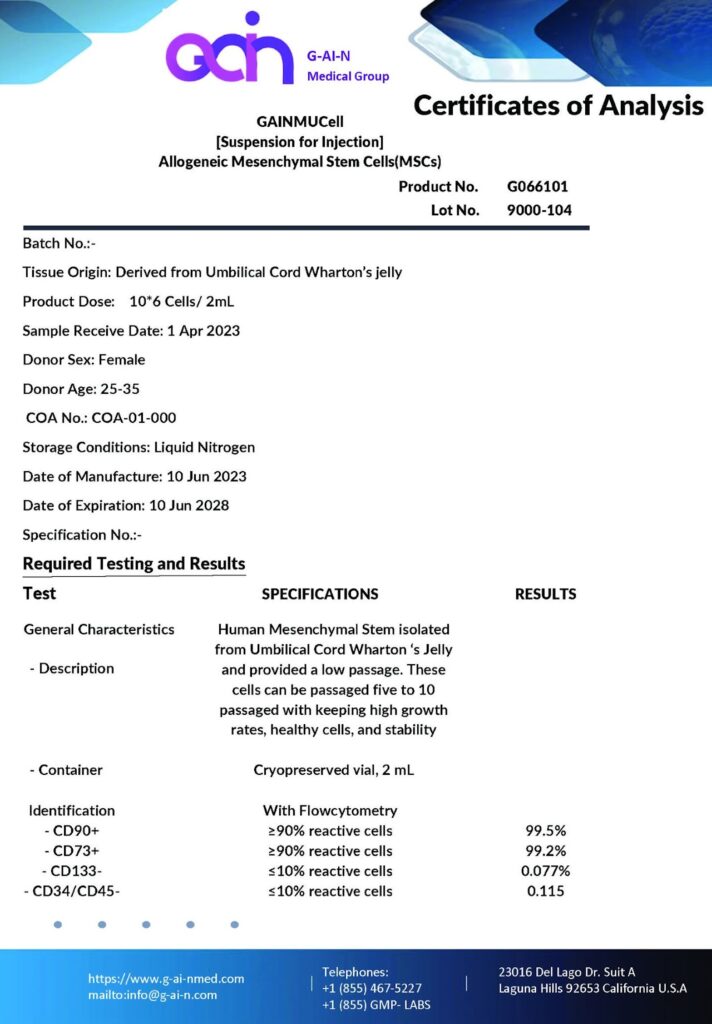
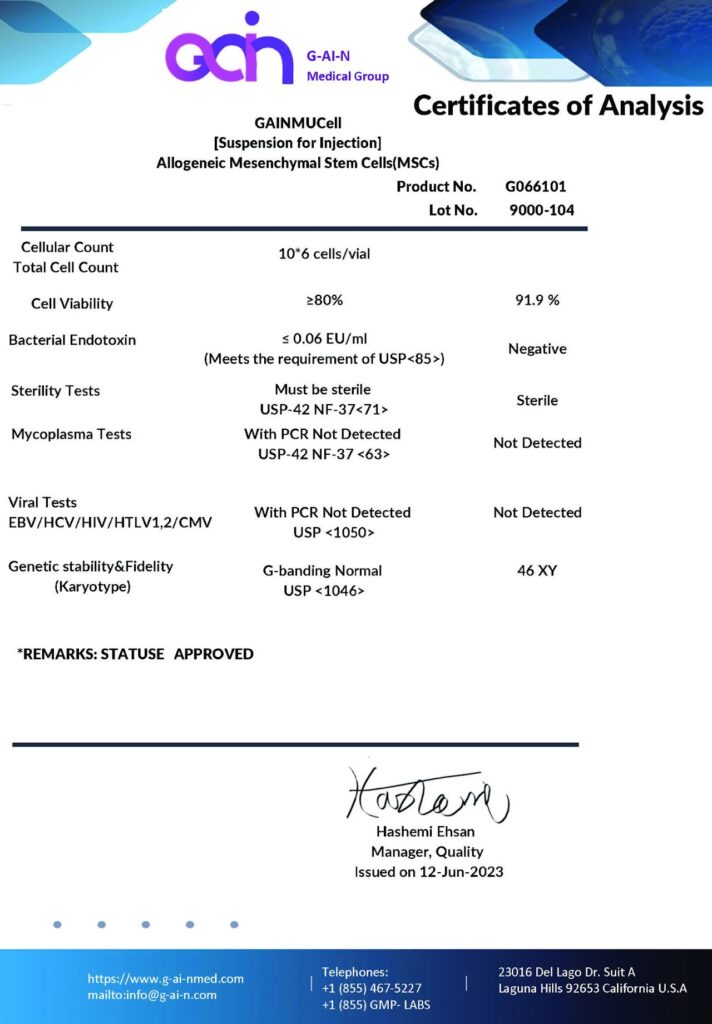
Analyzing the test results provided for GainMed’s stem cell products, we can provide a detailed, scientific explanation of the various sections and their significance:
Exceptional Purity and High Vitality Levels
The flow cytometry analysis, a critical method for assessing cell composition, indicates over 99% of cells express CD90 and CD73. These markers indicate the purity of mesenchymal stem cells and are essential for their functional efficacy in regenerative medicine. Additionally, a mere 0.077% of the cells express CD34, and 0.115% express CD45, markers typically associated with hematopoietic stem cells, confirming a minimal presence of non-MSCs and validating the exceptional purity of the MSC population.
Guaranteed Sterility
The results from sterility tests, including bacterial endotoxin levels, which are reported as negative (below the detection limit of ≤0.06 EU/ml), ensure that the stem cell products are free from bacterial contaminants and endotoxins, exceeding the stringent requirements of USP<85>. This level of sterility is crucial for preventing adverse reactions in patients upon the administration of stem cell products.
High Degree of Undifferentiation
The low passage number, indicated by the cells being only passaged one or two times, confirms that the cells have retained their undifferentiated state. This is critical as cells that are less differentiated are more effective for therapeutic purposes due to their higher regenerative capacity.
Exceptionally High MSC Content
The cellular count showcases that each vial contains 10^6 cells, with a viability of 91.9%, demonstrating that a high proportion of cells are alive and functional at the time of cryopreservation. This high count of viable cells is a strong indicator of the potential for successful clinical outcomes when these cells are used for therapeutic purposes.
Proof of Genetic Stability
The karyotype analysis showing a normal 46, XY genotype confirms the genetic stability of the stem cell batch. This normal karyotype is essential as it indicates that the cells do not carry chromosomal abnormalities that could lead to complications or reduce the therapeutic potential of the stem cells.
Summary
Every parameter measured in GainMed’s comprehensive testing protocol not only meets but often exceeds industry standards. The high mesenchymal stem cell purity, exceptional vitality, guaranteed sterility, maintained undifferentiation, and confirmed genetic stability all contribute to the superior quality of GainMed’s stem cell products. These results are a testament to GainMed’s meticulous approach to product quality, ensuring that their stem cell therapies are safe, effective, and ready to meet the rigorous demands of modern regenerative medicine.

